Alkyl Halides and Aryl Halides are classified as mono di or polyhalogen tri-tetra- etc compounds depending on the number of halogen atoms in their structures. The chemical reactivity of several alkyl halides is based on their structures and functions associated with them.
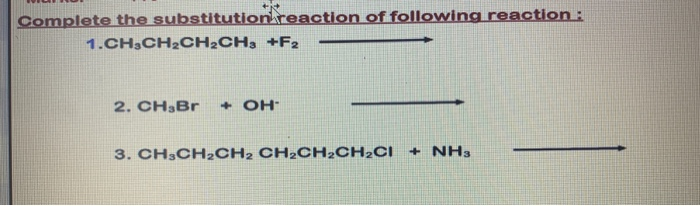
Solved 32 Draw Structures For These Alkyl And Aryl Halides Chegg Com
Find step-by-step Chemistry solutions and your answer to the following textbook question.
. Naming Halocarbons Organic molecules containing functional groups are given IUPAC names based on their main-chain alkane structures. In alkyl halides the halogen atom is bonded to an alkyl group R. _____ Brilliant Education Centre Villa No.
12 text -difluoro-3-iodocyclohexane text d. Classify each halo compound shown below as an alkyl vinyl or aryl halide. Aryl halides These are the compounds in which the halogen atom is directly.
If the compound is an alkyl halide indicate whether it is 1 2 or 3. Define functional group and name the group present in each of the following structures. The halogen atom is attached to a sp 2 hybridized carbon atom in aryl halides.
Recognize primary 1 o secondary 2 o and ter-tiary 3 o halides b. The carbon-halogen bond is stronger than that of alkyl halides due to the presence of ring electrons. An alkyl halide A on reaction with magnesium in dry ether followed by treatment with ethanol gave 2-.
BPK 400 Room 300 temperature 200 100 0 CH 3 X CH 32 CH X CH 322 CH CH X Gas Gas Chlorides Bromides Iodides Figure 121. With the exception of iodine these halogens have electronegativities significantly greater than carbon. B Aryl halides These are the compounds in which the halogen atom is bonded to the sp2-hybridised carbon atom of an aromatic ring.
Watch More Solved Questions in Chapter 22. Label each as primary secondary tertiary allylic or benzylic. We review their content and use your feedback to keep the quality high.
This problem has been solved. Draw the structures of the following alkyl halides. Who are the experts.
1122 text. Draw the structure of an alkyl halide that could be used in an E2 reaction to give the following alkene as the only alkene product. Until the late 1980s alkyl halides called chloro-.
Having learnt the classification of halogenated compounds let us now learn how these are named. Alkyl Aryl Halides. Fluorine chlorine bromine or iodine.
Experts are tested by Chegg as specialists in their subject area. 254Al Madeed Street Al Mamoura Doha Tel. And also if it is allylic or benzylic.
MAINIDEA Compare and contrast alkyl halides and aryl halides. Name these alkyl groups. The functional group of alkyl halides is a carbon-halogen bond the common halogens being fluorine chlorine bromine and iodine.
Name the type of organic compound each substance. Cl Cl 1-chloro-2-methylpropane 2R 3R 6S- 2 chloro 8 ethyl 36 dimethyl decane Cl Br Cl I F Br I Br F. Alkyl Halides RCX Alkyl halide X CC X Aryl halide Vinyl halide X F Cl Br I 101 Naming alkyl halides- Read 102 Structure of alkyl halides Table 101 Halomethane H3C-F H3C-Cl H3C-Br H3C-I Bond length pm 139 178 193 214 Bond strength KJmol 452 351 293 234 Dipole Moment 185 187 181 162 RCC _ Na CX H R H THF RCCC R H.
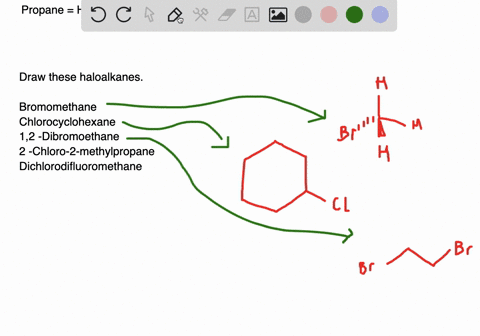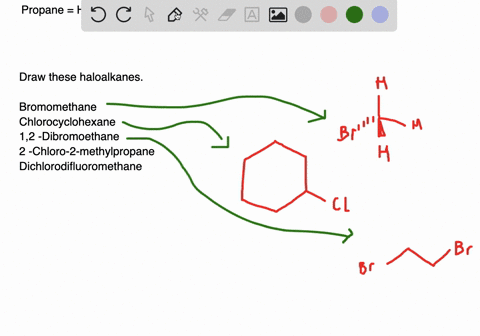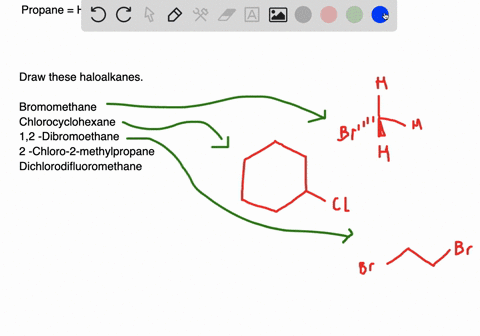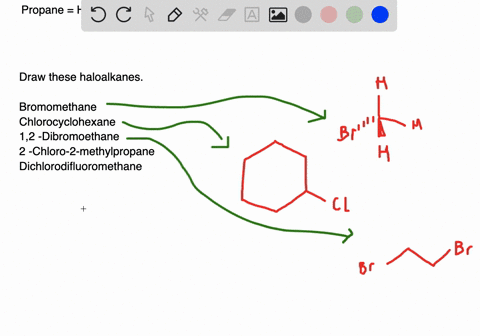
Name and write the structures of. Alkyl halide structures can be classified as primary secondary and tertiary. Draw structures for these alkyl and aryl halides.
Name and draw alkyl halides a. Which of these has weakest C CI bond. Give IUPAC names for the following alkyl halides.
Draw the structures of all the eight structural isomers that have the. For the alkyl halides a prefix. This is an unsaturated structure due to the presence of double bonds in the aromatic ring.
Chlorobenzene text b. 1 text -bromo-4-chlorohexane text c. Connection to Earth Science Alkyl halides are widely used as refrigerants.
Consider all the primary halides in this experiment and rank them in order of reactivity in each reagent. Write the name and draw the structure of the alkyl group that corresponds to 0202. Structural formula for an aryl halide is created by first drawing the aromatic structure and then replacing its hydrogen atoms with the halogen atoms specified as shown in Figure 223a.
FIGURE CANNOT COPY. Aryl halides also show dipole-dipole interactions. Aryl halides are always ringed structures.
13 text -dibromobenzene text e. Aryl halides tend to be less polar than alkyl halides since an sp 2 C is more electronegative than an sp 3 C What is the structure of aryl halide. Boiling points of haloalkanes Notice that three of these have bps below room temperature taken as.
Plan and predict the outcomes of the following chemi-cal reactons. In this lesson we will learn about alkyl halides and common. Alkyl halides have a linear or branched structure most of the times.
PHYSICAL PROPERTIES OF ALKYL HALIDES a Boiling point. For example At askIITians we provide you free study material on these topics so that you get all the professional help needed to get through IIT JEE and AIEEE easily. ALKYL HALIDES ARYL HALIDES Contents 1Nomenclature 2Classification 3Isomerism 4Method of preparation 5Properties 6Dihalides 7Grignard Reagent 8Aryl Halides The expert in anything was once a beginner Name.
Circle any organic halide below that can undergo a S N1 or S N2 type substitution reaction. Draw the structures of all the alkyl halides that will be used in this experiment. Draw structures for the following molecules.
The below chart shows the boiling point of some simple haloalkanes. An aryl halide is a molecule having a halogen atom attached to an sp2 hybridized carbon in an aromatic ring directly. 9 Lessons in Chapter 29.
Consequently this functional group is polarized so that the carbon is electrophilic and the halogen is nucleophilic as shown. Since the neutral bonding pattern for halogens is one bond and three lone pairs the carbon and halogen always share a single bond. The structural formula for an aryl halide is created by first drawing the aromatic structure and then replacing its hydrogen atoms with the halogen atoms specified.
The carbon-halide bond of alkyl halides has a low density of electrons compared to aryl halides. The word aryl means aromatic benzene ring and the word halide means a halogen of some sort. Recognize C α and C β in an alkyl halide 2.
Describe and compare the structures of alkyl halides and aryl halides. The common names of alkyl halides are derived by naming the alkyl group followed by the halide. Recognize alkyl halides as compared to vinyl and aryl halides c.
They form a homologous series represented by C n H. Bimolecular nucleophilic substitution S N2 b.

Solved Xex Dx 32 Draw Structures For These Alkyl And Aryl Chegg Com
Solved Draw Structures For These Alkyl And Aryl Halides Begin Array L Text A Chlorobenzene Text B 1 Text Bromo 4 Chlorohexane Text C 1 2 Text Difluoro 3 Iodocyclohexane
Solved Draw Structures For These Alkyl And Aryl Halides Begin Array L Text A Chlorobenzene Text B 1 Text Bromo 4 Chlorohexane Text C 1 2 Text Difluoro 3 Iodocyclohexane
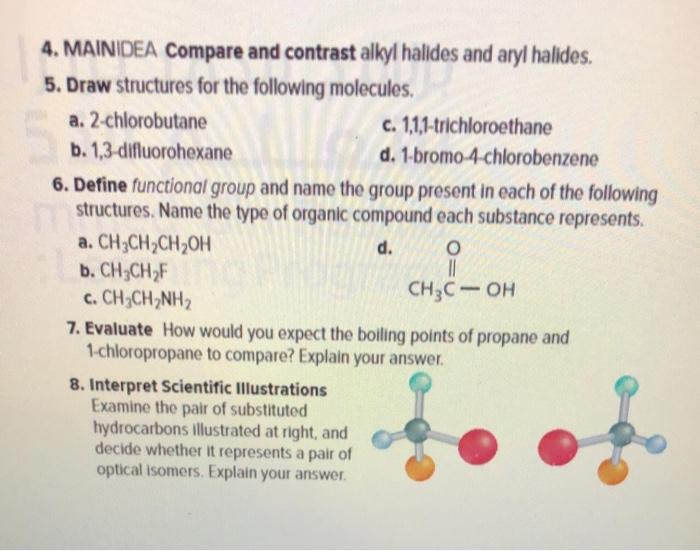
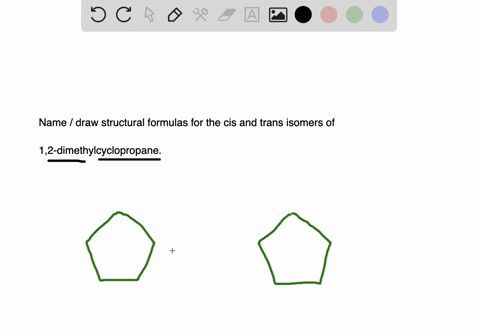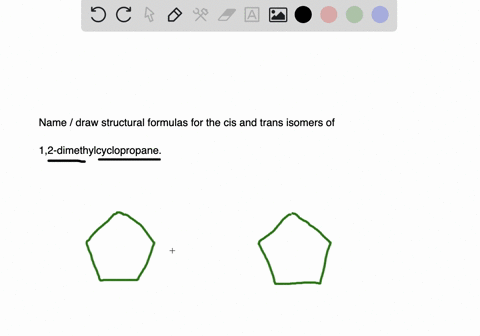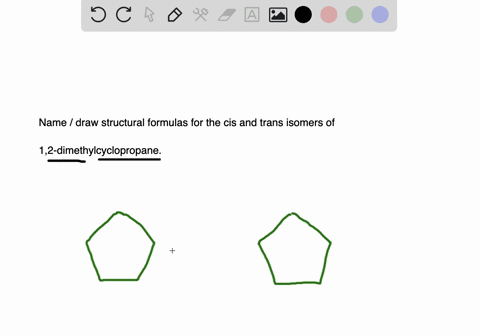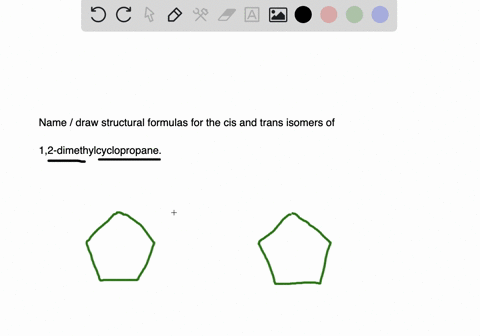
Solved 4 Maindea Compare And Contrast Alkyl Halides And Chegg Com
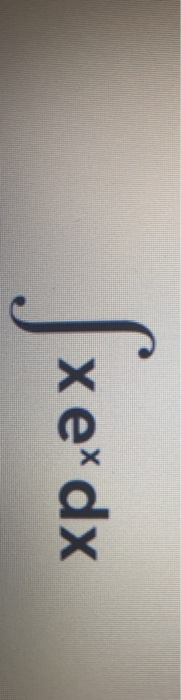
Solved Xex Dx 32 Draw Structures For These Alkyl And Aryl Chegg Com

Solved Draw Structures For These Alkyl And Aryl Halides Begin Array L Text A Chlorobenzene Text B 1 Text Bromo 4 Chlorohexane Text C 1 2 Text Difluoro 3 Iodocyclohexane

Solved Draw Structures For These Alkyl And Aryl Halides Begin Equation Begin Array L Text A Chlorobenzene Text B 1 Text Bromo 4 Chlorohexane Text C 1 2 Text

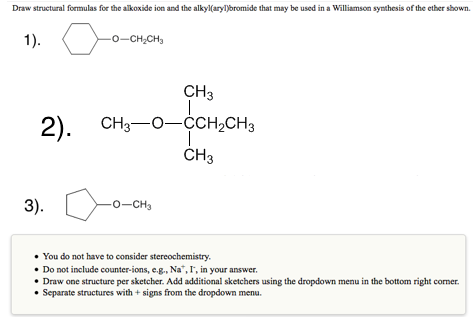
Solved Draw Structural Formulas For The Alkoxide Ion And The Chegg Com
0 comments
Post a Comment